Submitting Approvals
During the review process, most content under review will require formal approval from selected members of your team. Once you have recorded all your feedback and input for the content that is under review and have made all of the necessary changes, your document is ready to be submitted for approval. This is usually the last step of the review process before your content can be released.
Using Papercurve, the approval process is streamlined to increase productivity and ensure compliance. A unique feature of the Papercurve’s approval process is that is it built to in compliance with FDA CFR 21 Part 11 –which means all approvals are signed by an FDA compliant electronic signature!
How it Works
1. To submit your approval, navigate to the Papercurve dashboard, and load up your content.
2. Locate the Summary panel along the right-hand side, where you’ll find the Approvals tab which indicates how many approvals are remaining, and if your approval is requested for this document.

3. If your approval is requested, it will be shown in orange. Click on Submit Approval Now to submit your approval. As soon as you click on the tab, a drop-down menu of three approval options will appear:
Approved: this option gives your official approval, which means you are happy with the document and require no additional edits. The owner will receive your approval and can release the document into the approved library.
Approved with changes: this means that you require a few minor edits to the document, and once they are made, your approval will be complete. The document owner will be notified of the necessary changes, make the edits accordingly, and submit a new version. Your approval will be submitted only AFTER the changes have been implemented.
Request new version: When you request a new version, it indicates that you want the owner to incorporate the requested changes, upload a revised version, and then review the document again before giving your approval.
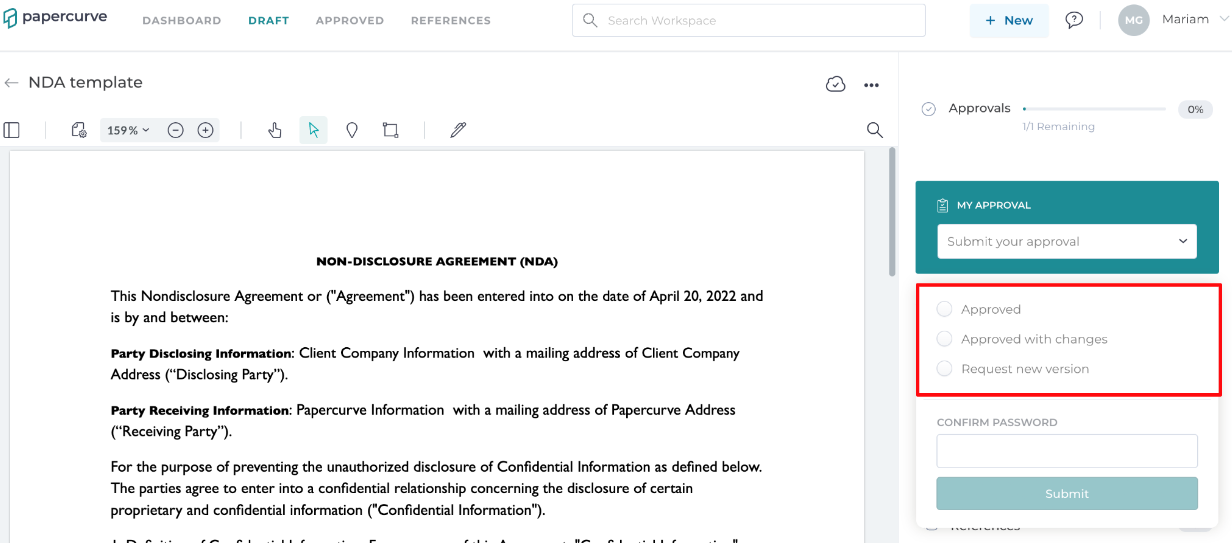
4. Once an approval status has been selected, you must enter your password to confirm and submit your selection.
This password is the same as your login credentials. Entering your password as part of your approval, ensures that you and your team is in compliance with the FDA’s guideline governing electronic records and electronic signatures

5. A green notification box in the top right corner will confirm you have submitted your approval for this document.

6. The Reviewer will then get an email notification of your actions.
Reset review status
Reviewers with status “Submit new version”
When a new version is uploaded, any reviewers with a status of “Submit new version” are automatically reset to “Waiting”. All users are notified that a new version has been uploaded.
Resetting someone’s review status
If you have uploaded a new version and some of your reviewers have already approved, you may want to reset their review status so that they can approve the newest version. You can reset a status by hovering over the reviewers name on the approvals page and clicking the “X” beside their status.

Manage approval
If you are interested in seeing the overall status of the outstanding approvals on your content, you can view the approval summary of other reviewers from the Summary Panel. Under Manage Approvals, you can add and remove reviewer as well as make changes to the Due Date and Library location.
Once all approvals has been processed, you may also release and revoke content as needed.




